
elegans sHsps are found in aggregated protein fractions of aged C. elegans sHsp genes are well characterized (iii) various C.
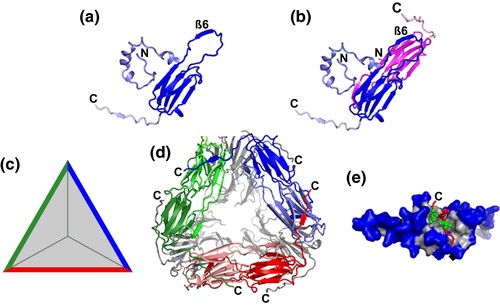
elegans expresses 16 sHsps, allowing us to explore the functional consequences of diversification and to identify common sequence features that might mediate sequestrase activity (ii) expression profiles of C. elegans as the model organism for the following reasons: (i) C. We focused on members of the sHsp family and chose C. This provides the unique possibility to screen proteins from other organisms for a potential sequestrase function. ΔΔbtn2Δ mutant cells are most affected and hardly grow at 30☌, representing the strongest phenotype of a sequestrase mutant reported so far. The lack of sequestrase activity in ΔΔbtn2Δ or ΔΔhsp42Δ cells therefore further reduces Hsp70 capacity, causing a temperature-sensitive growth phenotype ( Ho et al., 2019). The sequestration of soluble misfolded proteins into large inclusions by Btn2 or Hsp42 restricts their accessibility for Hsp70, thereby preventing the overload of the Hsp70 system.
#Chaperone proteins induced by heat shock free
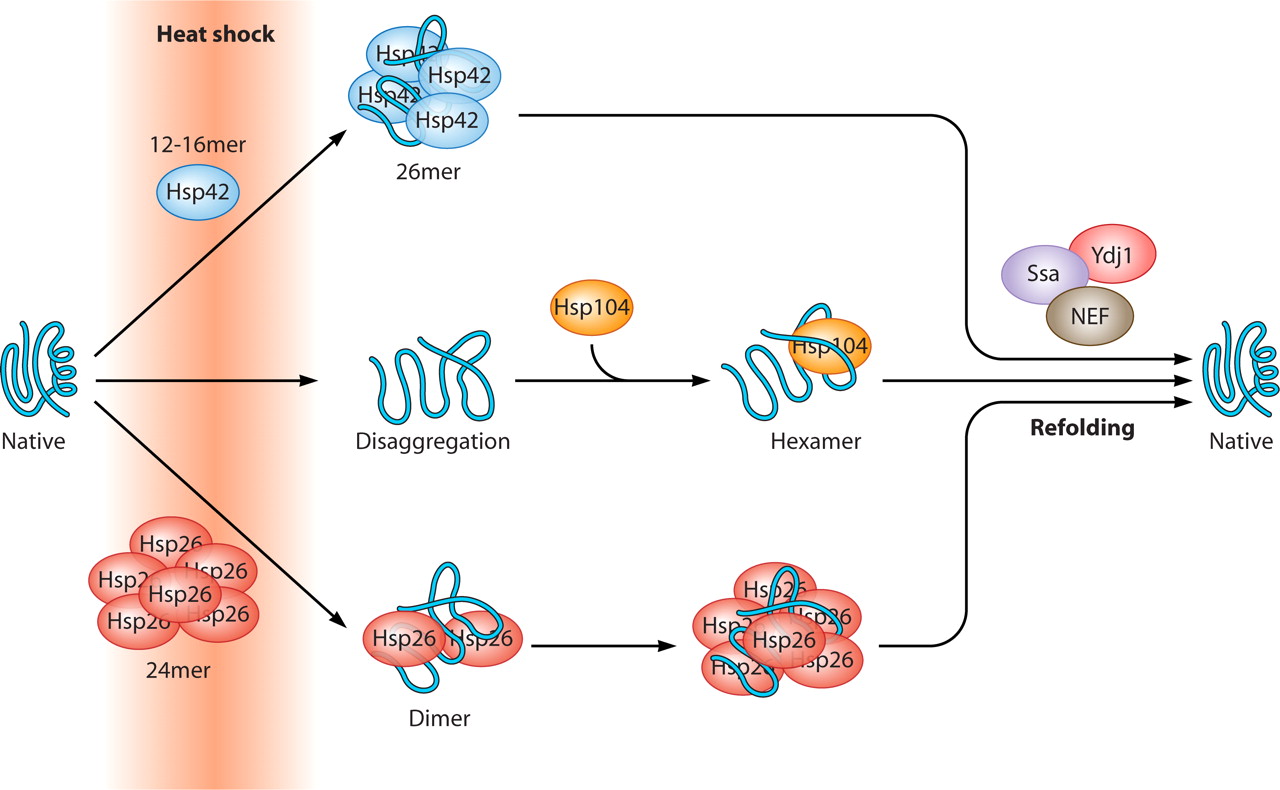
Both Fes1 and Hsp104 displace substrates from Hsp70, and in consequence, the levels of free Hsp70 become low in ΔΔ cells. cerevisiae fes1Δ hsp104Δ (termed ΔΔ) mutant cells, lacking the major nucleotide exchange factor (NEF) of Hsp70 in the nucleus, Fes1, and the AAA+ disaggregase Hsp104 ( Ho et al., 2019). We recently demonstrated that Btn2 and Hsp42 become essential for the growth of S. These findings demonstrate that sHsp sequestrase function is evolutionarily conserved and represents an originally cytoprotective activity of this chaperone family. coli IbpA and human HspB2/HspB3 are also able to partially rescue the defects of a yeast sequestrase mutant. elegans sHsps tested exhibit robust sequestrase activity and define specific sequence features of their disordered N-terminal extensions that are crucial for activity. This allowed us to screen for sHsps from diverse species that restore growth and misfolded protein sequestration in yeast. In this study, we overcame this obstacle by using the strong growth defects of a combination of yeast sequestrase and refoldase mutants. elegans: 16 members, human: 10 members Haslbeck and Vierling, 2015). This can be explained by compensatory activities of other proteostasis components ( Ho et al., 2019) and also by the large expansion of the sHsp family during evolution (e.g., C. The analysis of the function of sHsps in proteostasis networks is typically hampered by the absence of strong phenotypes of respective mutants. Cellular protection by sequestration of misfolded proteins is, therefore, an evolutionarily conserved activity of the sHsp family. elegans WT animals and are upregulated in long-lived daf-2 mutants, contributing to lifespan extension. Those sHsps buffer limitations in Hsp70 capacity in C. elegans’ sHsps harbor specific sequence features, including a high content of aromatic and methionine residues in disordered N-terminal extensions. We show that sequestration of misfolded proteins is an original and widespread activity among sHsps executed by specific family members.
We examined sHsps from Escherchia coli, Caenorhabditis elegans, and humans for their ability to complement the defects of yeast sequestrase mutants. Direct homologs of Hsp42 and Btn2 are absent in other organisms, questioning whether sequestration represents a conserved proteostasis strategy and, if so, which factors are involved.

The chaperone-mediated sequestration of misfolded proteins into inclusions is a pivotal cellular strategy to maintain proteostasis in Saccharomyces cerevisiae, executed by small heat shock proteins (sHsps) Hsp42 and Btn2.


 0 kommentar(er)
0 kommentar(er)
